Abstract
Background: Lenalidomide (LEN) monotherapy has been effective in extending progression free survival (PFS) and after myeloablative AuSCT in patients (pts) with multiple myeloma (MM) (Attal et al. NEJM 2012, McCarthy et al. NEJM 2012,). Elotuzumab (ELO), a humanized IgG1 immunostimulatory monoclonal antibody against signaling lymphocytic activation molecule F7 (SLAMF7), is FDA approved in combination with LEN and dexamethasone (DEX) for treatment of pts with MM who have received 1-3 prior therapies. The objective of this phase 2 trial is to evaluate the efficacy and safety of adding ELO to LEN as maintenance therapy post-myeloablative AuSCT. We report preliminary results of the primary (PFS) and secondary (overall survival and toxicity) endpoints.
Patients and Methods: Between 4/15/2015-1/27/2016, 28 evaluable pts were treated on 28 day cycles with ELO, 10 mg/kg iv weekly for cycles 1-2 and q2weeks for cycles 3-6, then 20 mg/kg once monthly for cycles 7+. Pts enrolled after 1/28/2016 (n=27 pts) have received ELO, 10 mg/kg IV weekly for cycles 1-2, and 20 mg/kg on day 1 from cycle 3 until progression. LEN has been dosed at 10 mg/day for cycles 1-3, with a dose increase to 15 mg/day at physician discretion starting with cycle 4, in the absence of non-hematologic toxicity > grade 1 and significant cytopenias (ANC < 1000/mL, platelet count < 100,000/ml). For the 1st 8 weeks, pts <75 yrs receive 28 mg of DEX 3-24 hours pre-infusion, while pts ≥75yrs receive 8mg; pts receive 4-10 mg iv DEX immediately pre-infusion for all cycles. Pts also receive herpes zoster prophylaxis and thromboprophylaxis commensurate with standard recommendations.
The primary endpoint of the study is PFS, defined as the time from AuSCT to clinical progression or death (whichever occurs first), or the time of last contact. Secondary objectives include best response, overall survival, incidence of second primary malignancies and adverse event (AE) profile. Total enrollment of 100 pts is planned. Pts will be followed for 48 months after the last patient is enrolled in the trial. Eligible pts had received no more than 2 lines of induction therapy, and were between 60-210 days post-AuSCT.
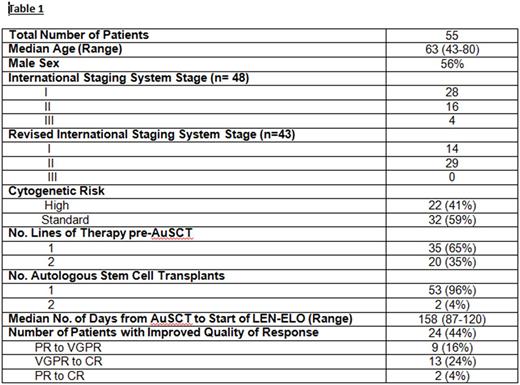
Results: Patients (n=55) have been treated for a median of 14 cycles (2-30). At study entry, 13 pts (24%) had complete response (CR), 27 (49%) had very good partial remission (VGPR), 14 (25%) had partial remission (PR) and 1 (2%) had minor remission (MR). Best response achieved to date on study is CR in 28 pts (51%), VGPR in 23 pts (42%) and PR in 4 pts (7%). For those who have converted to CR on study, median time to CR has been 5 months. Of 14 pts in CR who have been tested for MRD while on study, 13 are negative by flow cytometry (minimum of 2 million cells evaluated; sensitivity 10 -4-10-5). Two of these 13 have converted from VGPR to MRD negativity at 4 and 14 months on study, respectively.
With a median follow up of 21 months, 95% of pts (n=52) remain alive. Three pts (all with high risk disease) had disease progression at 4 (del 17p), 9 (t [4;14]), and 13 (gain 1q) months; of these, 2 have died of progressive disease while receiving salvage therapy. One patient, in VGPR, died on study after developing acute cerebral encephalopathy with refractory status epilepticus of unclear etiology. Two pts withdrew for logistical reasons; 2 have been taken off study per physician discretion (prolonged cytopenias (1), drug rash (1)).
Grade 3-4 Hematologic AEs (no. of pts) were: neutropenia 35% (16), thrombocytopenia 9% (4), febrile neutropenia 7% (3), and anemia 7% (3). Grade 3-4 non-Hematologic AEs (no. of pts): diarrhea 17% (8), fatigue 17% (8), pneumonia 15% (7), other infections 15% (7), peripheral neuropathy 11% (5), myalgias 9% (4), nausea/vomiting 7% (3), maculopapular rash 4% (2), dizziness 4% (2), edema 2% (1), memory impairment 2% (1). Renal cell carcinoma was diagnosed in 1 patient, 15 months after removal from study for disease progression.
Conclusions: Lenalidomide-elotuzumab is a well-tolerated maintenance therapy on which 44% of 55 pts have had improvement in quality of response while on therapy, including 28% who have converted to CR and 24% who have tested MRD negative. The number of pts who have experienced improvement on study may, in fact, be underestimated in this analysis due to elotuzumab interference with measurement on electrophoretic studies. Additional follow up is required to determine if the improved quality of responses translate into improvements in PFS and OS.
Thomas: Bristol Myers Squibb: Research Funding; Celgene: Research Funding. Shah: Kayopharm: Employment. Lee: Pimera Inc: Consultancy; Eutropics Pharmaceuticals: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees; Daiichi Sankyo: Research Funding; Takeda: Consultancy; Celgene: Consultancy. Manasanch: merck: Research Funding; adaptive biotechnologies: Consultancy; sanofi: Research Funding; celgene: Consultancy; takeda: Consultancy; quest diagnostics: Research Funding. Patel: Juno: Consultancy; Celgene: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding. Orlowski: BioTheryX: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal